Producing Electricity
Electricity can be produced by a number of methods including:
- Chemical reaction: These are normally cells containing chemicals that react with each other to produce electricity. Cells are usually combined together to make a battery. You are probably familiar with AA type cells that power your TV remote or toys, or the battery in a car used to start the engine.
- Solar power: Photons from the Sun displace electrons in the Cell. These electrons then move around a circuit.
- Mechanical Power: Loops of wire can be rotated through a magnetic field to generate electricity. Examples would include wind turbines or steam turbines that drive a generator.
AC / DC
Electricity doesn't always flow in one direction. It depends on the way it's produced.
Batteries and Solar Cells tend to make Direct Current (DC). DC always moves in the same direction all the time.
Mechanical generators tend to make Alternating Current (AC), but not always. AC switches direction, creating a voltage that goes between 1 and -1.
These trace two types of graph when recorded with a device called a cathode ray oscilloscope (CRO):

CRO - DC current trace

CRO - AC current trace
Chemical Reaction
Electricity can be produced by electrochemical reaction.
This is a process where one or more Voltaic Cells contain materials that react with each other to release negative electrons and positive ions. A Voltaic cell contains two electrodes: an Anode and a Cathode separated by a material called the Electrolyte. One or more Voltaic cells is known as a Battery.
The Anode and Cathode are different materials. The Anode collects -ve charge (electrons) and the Cathode attracts +ve charge. The electrons cannot move to the Cathode through the electrolyte and so move to it around a circuit if it's complete.
In a normal disposable battery, as the cell produces power the chemicals are changed into another chemical. Once the chemical has been fully transformed they cannot release any more electrons; the battery goes flat and ceases to produce power.
A rechargeable battery can be re-energized by passing an electric current through it in the opposite direction. This reverses the chemical process and restores the chemicals to their original state so that they can be used again.
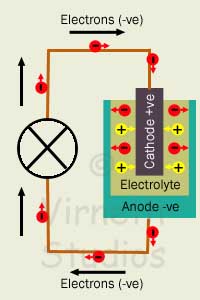
How a Battery Works

Some different types of Battery: Button, AA, PP3 and a 12 volt accumulator
Solar Power
The power of the sun can be harnessed directly using Solar Cells. Photovoltaic cells convert photons of light from the sun into electrons that are free to move around a completed circuit.
Photovoltaic cells are commonly assembled together into panels, which can then be grouped together to form large arrays that can produce reasonable amounts electricity, especially in the hot sunny regions of the world.
Special materials, called semiconductors, are used in the construction of PV cells. Silicone is commonly used because it has the right chemical properties. Its atomic structure is such that photons can knock electrons from their orbits around the nucleus.
The silicone is deliberately made impure by the addition of other substances to create two types of material: an N-Type semiconductor with spare electrons, and a P-Type semiconductor with holes for electrons to fill. These two types of semiconductors are arranged as two layers separated by an electric field. Some spare electrons fill some of the holes. When photons hits the cell electrons can be dislodged from their 'holes' and they move to the other side of the electric field. The electrons cannot move back across the field but if an alternative path, a circuit, is provided between the two layers they will take it to return to the 'hole'. This produces a useful current or electrons.
Dynamos
An electric current can be induced to flow through a conductor by moving it through a magnetic field. Scientists Michael Faraday and Joseph Henry first discovered this principle in 1831. A Dynamo uses this principle to convert mechanical energy into electrical energy.
Dynamos mainly produce direct current (DC), which isn't suitable for transmitting large amounts of power. Small dynamo's aren't very efficient. For these reasons dynamo's are rarely used these days. The most common application is probably for providing power for bicycle lights.
Alternators
Generator's and Alternator's use the same principle of induction that Dynamos use but produce AC electricity, rather than DC.
Click the Start / STOP buttons to see what happens when the coil rotates.
AC current is more common because the voltage can be stepped up and down using of transformers. This allows very high voltages to be transmitted at a low impedance, which reduces high energy losses.
All content Copyright © Virneth Studios 2005-2011, All Rights Reserved